Tofacitinib Cream for Treatment of Vitiligo
Tofacitinib Cream for Treatment of Vitiligo
By Casey Battersby, Pharm.D
Candidate 2020
July 3rd, 2019
CareFirst Specialty Pharmacy
What is Vitiligo?
Vitiligo is a skin disease that causes the loss of skin color in patches on different parts of the body. This is not a contagious or life-threatening condition, however, it can be stressful for a patient to experience and have negative effects on a person’s self-esteem.
Vitiligo is most commonly seen as patchy losses of skin pigmentation and it can be seen anywhere on the body. Patients with vitiligo may also experience premature whitening or graying of their scalp, eyelashes, eyebrows, or facial hair. Some patients may also experience loss of colors in the mucous membrane tissues that line the inside of the mouth and the nose and some even experience a loss of or change of color of the retina.
This disease usually presents in a patient by the age of 20 and the areas of depigmentation increase over time. There are three types of vitiligo: generalized, segmental, and localized. Generalized vitiligo is the most common type, it can occur anywhere on the body, and usually occurs symmetrically. Segmental vitiligo involves only one side or a part of the body. It also tends to occur at a younger age and progresses for one to two years before stopping. Localized vitiligo, also referred to as focal vitiligo, occurs in only one or a few areas of the body.
What Causes Vitiligo?
The pathophysiology of vitiligo is not completely understood. On a cellular level, vitiligo occurs when pigment-producing cells (melanocytes) in the epidermis layer of the skin stop producing melatonin. Melatonin is the pigment in your body that determines the color of your skin, hair, and eyes and also helps protect your skin from ultraviolet light. When no melatonin is being produced, the affected area of the skin begins to turn white. Vitiligo is the spreading of that loss of color due to loss of melatonin production.
Why the melanocytes die and fail to produce melatonin is the component of vitiligo that is not entirely known. Doctors commonly attribute the disease to be one of the following:
- An immune disorder where your immune system attacks and destroys that melanocytes in your epidermis.
- Hereditary disorder in patients with a family history.
- The result of a trigger event, such as sunburn, emotional distress, or exposure to industrial chemicals.
However, there is no definitive proof that any of these reasons are the true cause of vitiligo.
How is Vitiligo Treated?
There are currently no cures for vitiligo, however, there are treatments that can help to potentially halt the progression of the disease and others that can help restore pigmentation to the affected areas. Topical, systemic, and light-based therapies are available for the stabilization and repigmentation of vitiligo.
For stabilization of rapidly progressing disease, low dose systemic corticosteroids, such as oral prednisone or dexamethasone, are the first-line therapy, however, studies supporting this are limited and uncontrolled.
For localized disease, mid- to high-potency topical corticosteroids are the first-line therapy. These agents include betamethasone, mometasone, and clobetasol. Typically, these topicals are used for two months on, one month off. For vitiligo that is localized to the face, topical calcineurin inhibitors, such as tacrolimus and pimecrolimus, are the preferred first-line therapy over topical corticosteroids due to the high chance of atrophy in that area. Targeted phototherapy is also an option for localized disease in patients that don’t respond to topical therapies.
For patients that experience vitiligo on 10% or greater of their body surface area, the first-line recommended therapy is Narrowband UVB phototherapy. Patients with extensive disease (> 40% of body surface area affected) that don’t wish to undergo this phototherapy may opt for depigmentation therapy with hydroquinone instead.
Other topical agents that are used for vitiligo that have not been thoroughly tested include calcipotriol and tacalcitol, vitamin D3 analogs. PUVA photochemotherapy has also been used for repigmentation therapy but is mostly replaced by the NB-UVB phototherapy due to decreased adverse effects. Vitamin C, vitamin B12, alpha-lipoic acid, and Ginkgo biloba are also used as adjunct therapies but are not exclusively studied.
Experimental Treatments
There are currently multiple experimental studies for the treatment of vitiligo. Afamelanotide is a synthetic analog of naturally occurring alpha-melanocyte-stimulating hormone (MSH) that is administered as a subcutaneous, bioresorbable implant that promotes melanocyte proliferation and melanogenesis. It was studied for safety and efficacy in combination with NB-UVB phototherapy and was found to induce deep repigmentation as well as diffuse hyperpigmentation in all cases studied.
Prostaglandin E2 (PGE2) is being studied for the treatment of localized, stable vitiligo. PGE2 helps control the proliferation of melanocytes by means of stimulant and immunomodulatory effects. Studies of the PGE2 0.23 mg/g gel have shown significant cases of repigmentation.
Bimatoprost is a synthetic analog of prostaglandin F2-alpha that is FDA approved for topical treatment of glaucoma and hypotrichosis. One of the side effects of this medication is hyperpigmentation of periocular skin due to increased melanogenesis induced by the drug. Because of this, it is being studied for use in vitiligo and thus far has proven to be most beneficial in facilitating the repigmentation of the face.
The last drug being studied for use in vitiligo is Ruxolitinib, a janus kinase 1 and 2 inhibitor. This drug is approved for the treatment of intermediate to high-risk myelofibrosis and polycythemia vera. It is currently in clinical trials to be used as a cream for the treatment of vitiligo. The best response seen throughout these trials has been in the treatment of facial vitiligo.
Tofacitinib
Tofacitinib is a janus kinase (JAK) inhibitor that works to decrease the intracellular activity of immune cells by preventing cytokine or growth factor-mediated gene expression. It is currently used for the treatment of psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.
In the United States, the approved dosage forms of Tofacitinib are an oral tablet in either 5 or 10 mg and an extended-release tablet that is 11mg.
Common adverse events associated with Tofacitinib include upper respiratory tract infections, headache, diarrhea, and cold symptoms. This drug has also been seen to cause fever, rash, and vomiting. Patients on Tofacitinib need to be monitored for bone marrow suppression and potential infections due to the suppression of immune cells in the body.
Tofacitinib for Vitiligo
Tofacitinib is currently being studied for use in Vitiligo. One of the central pathways of vitiligo that seems to be important to the disease progression is the interferon-gamma signaling pathway. Involved in this pathway are the JAK receptors. Because of this, the use of a JAK receptor can be used to block the pathway and therefore block the progression of the disease and help induce repigmentation.
Efficacy studies have been done of Tofacitinib oral for treatment of vitiligo. A small, retrospective study done in 2017 of the use of oral Tofacitinib for vitiligo studied the use in 10 patients. Only five of these patients had a response and it was noted that response was better on the sun-exposed areas of the skin. Because of this, it was recommended that Tofacitinib be used in combination with phototherapy.
A more recent article outlining two successful case reports show more promise for the use of Tofacitinib in vitiligo. Case one reports a patient used Tofacitinib 5mg twice daily concomitantly with full-body NB-UVB phototherapy on her face. Vitiligo had affected 75% of her face and after three months of treatment, she had regained pigmentation in almost her entire face.
Case two outlines a male patient that had pigmentation loss in 90% of his face. He also used Tofacitinib 5mg twice daily with full-body NB-UVB phototherapy. After three months, he regained 50% repigmentation in his face and after six months he regained 75% repigmentation.
All of these studies show that concomitant use of phototherapy with Tofacitinib yielded the best results. Additionally, repigmentation mostly occurred on the facial reasons of these patients and there was very little to no repigmentation of the rest of the body. Due to this fact, studies involving Tofacitinib as a topical cream have emerged in the last year.
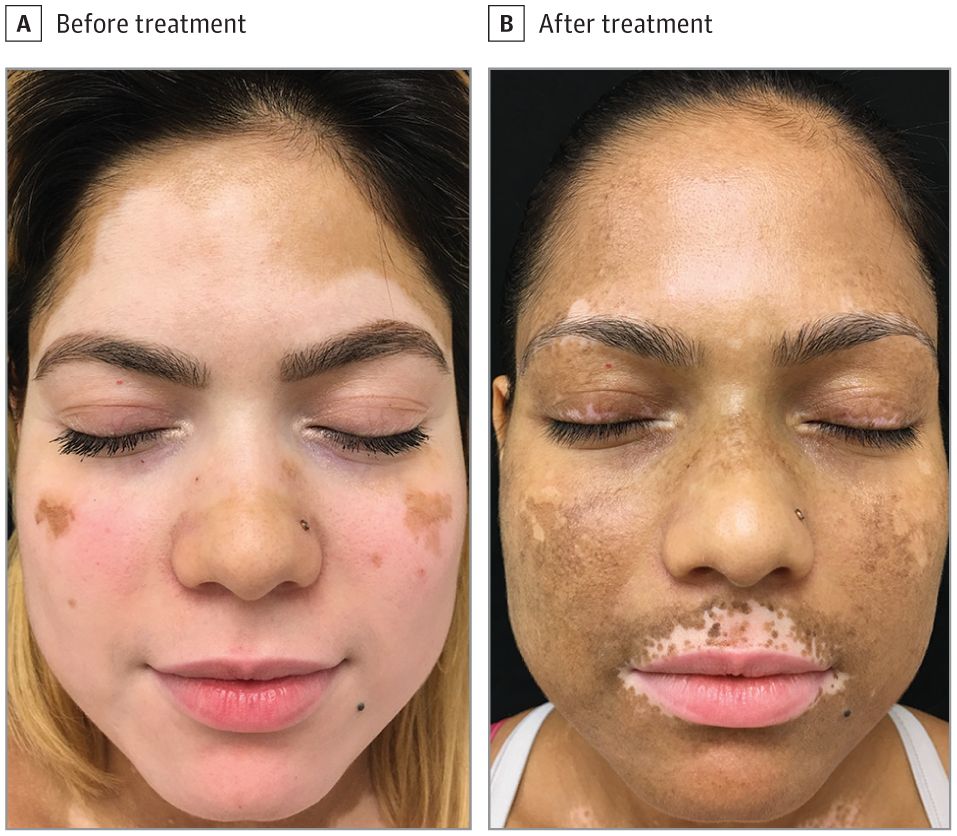
Topical Tofacitinib has been studied the past couple years for use in alopecia however there are not yet published studies about use for vitiligo. According to clinicltrials.gov, a study concluded last year on the use of topical Tofacitinib in vitiligo, however, the article outlining the results of the study has not yet been published. As previously mentioned, another JAK inhibitor, Ruxolitinib, has been studied for topical use in vitiligo. Because of this and the results of the trials involving oral Tofacitinib, there is currently a theoretical use of topical Tofacitinib for use in vitiligo.
Tofacitinib is not yet commercially available as a topical agent. If you and your doctor wish to try topical Tofacitinib, you can order it from a specialty compounding pharmacy such as CareFirst Specialty Pharmacy. We are located at 2200 Garry Road, Suite 1, Cinnaminson, New Jersey 08077.
References
- Yaghoobi R, Omidian M, Bagherani N. Vitiligo: a review of the published work. J Dermatol 2011; 38:419.
- Faria AR, Tarlé RG, Dellatorre G, et al. Vitiligo--Part 2--classification, histopathology and treatment. An Bras Dermatol 2014; 89:784.
- Falabella R, Barona MI. Update on skin repigmentation therapies in vitiligo. Pigment Cell Melanoma Res 2009; 22:42.
- Njoo MD, Spuls PI, Bos JD, et al. Nonsurgical repigmentation therapies in vitiligo. Meta-analysis of the literature. Arch Dermatol 1998; 134:1532.
- Grimes PE. Vitiligo. An overview of therapeutic approaches. Dermatol Clin 1993; 11:325.
- Kim SM, Lee HS, Hann SK. The efficacy of low-dose oral corticosteroids in the treatment of vitiligo patients. Int J Dermatol 1999; 38:546.
- Taieb A, Alomar A, Böhm M, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013; 168:5.
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev 2015; :CD003263.
- Njoo MD, Bos JD, Westerhof W. Treatment of generalized vitiligo in children with narrow-band (TL-01) UVB radiation therapy. J Am Acad Dermatol 2000; 42:245.
- Khullar G, Kanwar AJ, Singh S, Parsad D. Comparison of efficacy and safety profile of topical calcipotriol ointment in combination with NB-UVB vs. NB-UVB alone in the treatment of vitiligo: a 24-week prospective right-left comparative clinical trial. J Eur Acad Dermatol Venereol 2015; 29:925.
- Gupta D, Kumari R, Thappa DM. Depigmentation therapies in vitiligo. Indian J Dermatol Venereol Leprol 2012; 78:49.
- Lim HW, Grimes PE, Agbai O, et al. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: a randomized multicenter trial. JAMA Dermatol 2015; 151:42.
- Kapoor R, Phiske MM, Jerajani HR. Evaluation of safety and efficacy of topical prostaglandin E2 in treatment of vitiligo. Br J Dermatol 2009; 160:861.
- Kapur R, Osmanovic S, Toyran S, Edward DP. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol 2005; 123:1541.
- Rothstein B, Joshipura D, Saraiya A, et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J Am Acad Dermatol 2017.
- Tofacitinib. Lexicomp Online, Hudson, Ohio: Wolters Kluwer Clinical Drug Information, Inc.; 2019; July 2, 2019.
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the janus kinase inhibitor, tofacitinib, may require concomitant light exposure. J Am Acad Dermatol. 2017 Oct; 77(4): 675–682.e1.
- Kim SR, Heaton H, Liu LY, King BA. Rapid Repigmentation of Vitiligo Using Tofacitinib Plus Low-Dose, Narrowband UV-B Phototherapy. JAMA Dermatol. 2018;154(3):370–371. doi:10.1001/jamadermatol.2017.577
- https://www.aad.org/public/diseases/color-problems/vitiligo
- https://www.mayoclinic.org/diseases-conditions/vitiligo/symptoms-causes/syc-20355912
- http://www.avrf.org/facts/cause-of-vitiligo.html
- https://www.dermatologytimes.com/article/jak-inhibitors-offer-hope-vitiligo-patients


Comments