LDN Prescriber Information
Naltrexone is often classified as an opiate antagonist. However, at low-doses, Naltrexone acts to inhibit certain inflammatory pathways, which involve Toll-Like Receptors (TLRs). Naltrexone’s traditional use is in treating addiction to opiate drugs, such as heroin or morphine, or to treat acute overdose of these opioids. The daily dose of Naltrexone used for this purpose is usually between 50 mg (moderate dose naltrexone) to 300mg (high dose Naltrexone). Similar levels of Naltrexone are using for acute dosing.
In the USA, Low-dose Naltrexone (LDN) has been used for the treatment of autoimmune diseases since 1985. Although LDN has used at low-dosages for many years by patients with autoimmune disorders, the occurrence of significant introductory-effects of LDN, as well as long-term side-effects of LDN, have not been extensively studied.
A widely-used LDN dosage protocol was first developed in the 1980s by the late Dr. Bernard Bihari, who was qualified in Neurology, Internal Medicine, and Psychiatry.
How Naltrexone Works
As of 2016, LDN is most commonly being used for Chronic Fatigue, Multiple Sclerosis, CFS/ ME, autoimmune thyroid diseases, and various cancers. Many autoimmune diseases seem to respond to LDN.
This is a wide range of diseases. Some Clinicians may find it difficult to accept that a single drug can have a positive effect on a wide range of pathologies. However, LDN is a known to be a potent antagonist to (a) certain opioid receptors, as well as (b) a wide range of inflammation-mediating Toll-Like Receptors (e.g. TLR4, TLR7/8/, TLR9).
It is important to emphasize that Naltrexone (the drug in LDN prescriptions) is produced in a 50:50 mixture of two different chemical-shapes (called isomers). It has been recently discovered that one chemical-isomer binds to immune cells, whilst the other chemical-isomer binds to opioid receptors.
Although having the same atomic-composition, the two chemical-isomers of Naltrexone have very different therapeutic activities.
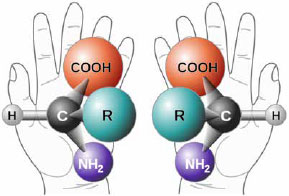
The LEVO (left-handed) version of Naltrexone blocks opiate receptors.
The DEXTRO (right-handed) version blocks receptors on immune cells. These include "Toll-Like Receptors" (TLRs), which are heavily involved in immunity. LDN is a potent antagonist of TLR-4, as well as an antagonist of TLR7/8 and TLR-9 (ref 10).
For clinicians interested in reading more about the pharmacology behind this, there is a published resource available here:
https://www.ldnresearchtrust.org/ldn-book
Summary of Mechanism of Action
Levo-Naltrexone is an antagonist for the Opiate/Endorphin receptors
- This causes increased Endorphin release
- Increased Endorphins modulate the immune response
- This reduces the speed of unwanted cells growing
Dextro-Naltrexone is an antagonist for at least one, if not more immune cells
- Antagonises "TLR", thereby suppressing cytokine modulated immune system
- Antagonises TLR-mediated production of NF-kB – thereby reducing inflammation, potentially down-regulating oncogenes
Taking Naltrexone in larger doses of 50-300mg seems to negate the immunomodulatory effect by overwhelming the opioid receptors. The Naltrexone dose must be in the range of 0.5mg, usually maxing out its benefit at 4.5mg in clinical experience. However, some individuals experience positive clinical effects at lower doses, others at higher doses than 4.5mg.
Side Effects
- Many patients who start LDN do not experience any severe side effects.
- As mentioned earlier, your symptoms may become worse. In Multiple Sclerosis (MS), this can be characterized by increased fatigue or increased spasticity. In CFS/ME, this can be the onset of apparent flu-like symptoms.
- LDN can cause sleep disturbances if taken at nighttime – this is most likely because of the increase in endorphin release. These disturbances can take the form of vivid dreams, or insomnia.
- In various studies (and anecdotal accounts), the number of T-Lymphocytes has been shown to dramatically increase when a patient starts on LDN. This may account for some of the benefits patients feel when they are being treated for an autoimmune disease or cancer. This has not been directly evidenced in Multiple Sclerosis.
- Clinical experience shows that in less than ten percent of cases treated, increased introductory symptoms may be more severe or more prolonged than usual, lasting sometimes for several weeks. Rarely, symptoms may persist for 2-3 months before the appropriate beneficial response is achieved.
- If side effects are troublesome, then reducing your dose by 50% for 7 days, before increasing it again, is a good idea.
- Very rarely, some patients experience gastrointestinal (GI) side effects, such as nausea and or constipation/diarrhea. The reason for this is currently unknown. The side effects may be due to the presence of large numbers of delta opiate receptors in the intestines. Patients experiencing GI side-effects can request LDN Sublingual Drops, which transfer the LDN directly into the bloodstream – avoiding the GI tract.
Types of LDN
- Liquid
- Oral Liquid Formulation at 1mg/1ml is the most commonly used type of LDN. It is taken daily and dosed using a baby oral syringe. It does not contain very high amounts of lactose or any other excipient known to cause hypersensitivity. The base is similar to children’s cough syrup – so it is quite palatable. Because there are so few preservatives, it should be stored in the fridge.
- Capsules
- For patients who the liquid would be impractical, there are capsules available in 3mg and 4.5mg strengths. Other strengths are available from different manufacturers – but these are made on a case by case basis. These have up to 12 months of stability data and can be stored anywhere. They contain no lactose filler and are instant release.
- Sublingual Drops
- Sublingual drops are designed for patients who are having problems taking the medication orally, or for people who want to guarantee the fastest delivery of the drug into their bloodstream. A number of drops are placed under the tongue from a dropper bottle and dose is increased and decreased by the number of drops taken. There are very few excipients in this product; only a trace amount of lactose, and a small amount of glycerol.
- Cream
- LDN Cream in 0.5mg/ml is available for application to the skin. This is helpful for children, or for patients allergic to colorants, or other excipients, in different formulations of LDN. LDN Cream is more expensive than oral LDN, and a typical prescription usually lasts for only 28 days.
Intrinsic Toxicity of the Drug
Naltrexone, in full doses of 50-300mg, has been shown to transiently increase liver enzymes. Patients being prescribed Naltrexone for addictions must have liver function tests performed before initiating therapy.
This is not necessary with LDN – as the dose is much smaller. However, patients with advanced liver failure should consult their General Practitioner (GP) before considering treatment.
Patients with renal or liver failure should only start treatment after a consultation with their own GP or specialist and should be monitored during the treatment initiation period. It is normal for people with poor renal or liver function to experience a transient elevation – but this usually resolves after a few weeks.
Contraindications and Special Precautions
- LDN is compatible with most other therapies. LDN does not directly interact with steroids. However, LDN can negate the effect of opiate-based painkillers. Patients should give their doctor a full drug history before starting therapy.
- Patients who are taking multiple medications and/or herbal medicines – especially those with cancer or advanced disease, should take careful advice from a qualified doctor or pharmacist before initiating LDN.
Key Clinical Studies
- Low Dose Naltrexone (LDN) has been the subject of much debate but actually very few clinical trials. Dr. Ian Zagon from Penn State University has been studying LDN for over 20 years and conducted many preclinical studies investigating LDN in cancer and in the animal model of MS (1,2). He has also been involved in two clinical studies into Crohn’s disease with his colleague, Dr. Jill Smith, from Penn State. These two researchers have demonstrated a significant improvement in symptoms and in bowel mucosal appearance with LDN treatment (3,4). In the RCT, LDN patients were twice as likely to have a 70-point decline in the Crohn’s Disease Activity Index. 78% of the LDN group achieved an endoscopic response compared to 28% with placebo.
- Dr. Jarred Younger from Stanford University has studied LDN in Fibromyalgia, firstly in a small pilot study, and more recently in a yet to be published randomized controlled trial. The pilot study showed significant improvement in symptoms of pain in these patients (5).
- Multiple Sclerosis (MS) is one of the areas where LDN has been used the most frequently. There are three published studies, one in primary progressive MS (6) and two on quality of life (7,8). The results of the two studies were positive with improved quality of life in one and reduced spasm in the PPMS study. The third showed no significant difference between the treatment and placebo groups but found the treatment to be safe. A review of the available studies into LDN and MS was published in 2009 (9). All studies have confirmed the safety of the drug and there is enough positive evidence to merit greater investigation.
Key References
- Rahn KA, McLaughlin PJ, Zagon IS. Prevention and diminished expression of experimental autoimmune encephalomyelitis by low dose naltrexone (LDN) or opioid growth factor (OGF) for an extended period: Therapeutic implications for multiple sclerosis. Brain Res. 2011 Mar 24;1381:243-53. Epub 2011 Jan 20.
- Donahue RN, McLaughlin PJ,Zagon IS The opioid growth factor (OGF) and low dose naltrexone (LDN) suppress human ovarian cancer progression in mice. Gynecol Oncol. 2011 Aug;122(2):382-8. Epub 2011 Apr 30.
- Smith JP, Stock H, Bingaman S, Mauger D, Rogosnitzky M, Zagon IS Low-dose naltrexone therapy improves active Crohn’s disease. Am J Gastroenterol. 2007 Apr;102(4):820-8. Epub 2007 Jan 11.
- Smith JP, Bingaman SI, Ruggiero F, Mauger DT, Mukherjee A, McGovern CO, Zagon IS. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial.Dig Dis Sci. 2011 Jul;56(7):2088-97. Epub 2011 Mar 8.
- Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009 May-Jun;10(4):663-72. Epub 2009 Apr 22.
- Gironi M, Martinelli-Boneschi F, Sacerdote P, Solaro C, Zaffaroni M, Cavarretta R, Moiola L, Bucello S, Radaelli M, Pilato V, Rodegher M, Cursi M, Franchi S, Martinelli V, Nemni R, Comi G, Martino G. A pilot trial of low-dose naltrexone in primary progressive multiple sclerosis. Mult Scler. 2008 Sep;14(8):1076-83.
- Cree BA, Kornyeyeva E, Goodin DS Pilot trial of low-dose naltrexone and quality of life in multiple sclerosis. Ann Neurol. 2010 Aug;68(2):145-50.
- Sharafaddinzadeh N, Moghtaderi A, Kashipazha D, Majdinasab N, Shalbafan B. The effect of low-dose naltrexone on quality of life of patients with multiple sclerosis: a randomized placebo-controlled trial. Mult Scler. 2010 Aug;16(8):964-9. Epub 2010 Jun.
- Gilhooly TC Low-dose naltrexone as a treatment for multiple sclerosis British Journal of Neuroscience Nursing, Vol. 5, Iss. 11, 13 Nov 2009, pp 494.
- Cant R, Dalgleish AG, Allen RL. Naltrexone inhibits IL-6 and TNF-alpha production in human immune cell subsets following stimulation with ligands for intracellular Toll-Like receptors. Front Immunol. 2017 July; 8:809.


Comments